France Pharmacokinetics Services Market Size, Share, and COVID-19 Impact Analysis, By Drug Type (Small Molecules and Large Molecules), By Application (SMEs and Large Enterprise), and France Pharmacokinetics Services Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareFrance Pharmacokinetics Services Market Insights Forecasts to 2035
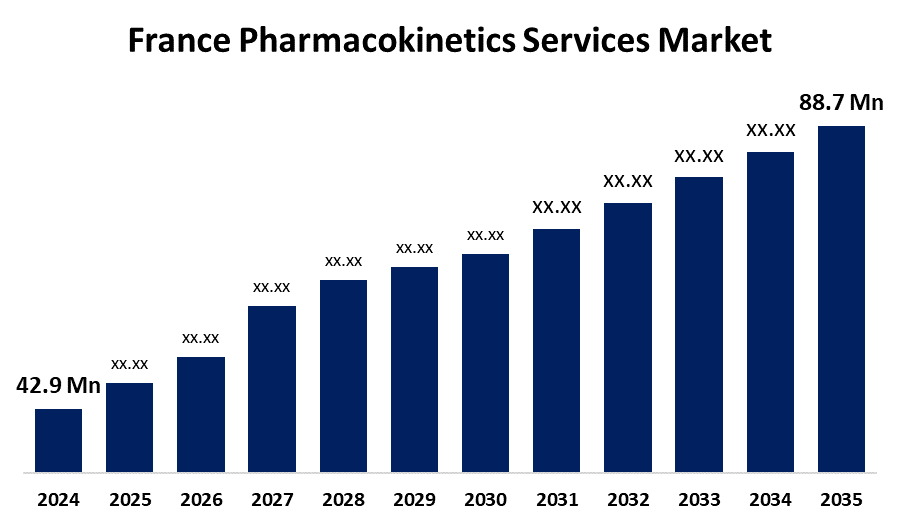
- The France Pharmacokinetics Services Market Size was Estimated at USD 42.9 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 6.83% from 2025 to 2035
- The France Pharmacokinetics Services Market Size is Expected to Reach USD 88.7 Million by 2035
Get more details on this report -
The France Pharmacokinetics Services Market Size is anticipated to reach USD 88.7 Million by 2035, growing at a CAGR of 6.83% from 2025 to 2035. The France pharmacokinetics services market is driven by increasing R&D investments, regulatory support, and demand for personalized medicine. These factors enhance drug development efficiency and patient-specific treatment strategies.
Market Overview
The France pharmacokinetics services market focuses on analyzing the absorption, distribution, metabolism, and excretion (ADME) of pharmaceutical compounds. These services are essential in the drug development process, ensuring accurate dosage formulation, safety assessments, and therapeutic effectiveness. Pharmacokinetics studies help researchers understand how drugs behave in the human body, providing vital data required for clinical trials and regulatory approvals. In France, the market encompasses a broad range of services, including in vitro and in vivo studies, bioanalytical testing, and modeling and simulation of pharmacokinetic profiles. The market includes collaborations between pharmaceutical companies, contract research organizations (CROs), and academic research centers. France’s strong scientific and regulatory environment supports comprehensive pharmacokinetics research, ensuring high-quality data generation in compliance with international standards. With a structured approach to drug evaluation, pharmacokinetics services contribute to the safe and effective development of pharmaceuticals, playing a crucial role in advancing healthcare and therapeutic innovation across the country.
Report Coverage
This research report categorizes the market for the France pharmacokinetics services market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the France pharmacokinetics services market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the France pharmacokinetics services market.
France Pharmacokinetics Services Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 42.9 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | CAGR Of 6.83% |
| 2035 Value Projection: | USD 88.7 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 247 |
| Tables, Charts & Figures: | 101 |
| Segments covered: | By Drug Type and By Application |
| Companies covered:: | Charles River Laboratories, Laboratory Corporation of America (Labcorp) Drug Development, Syneos Health, Quotient Sciences, Eurofins Scientific, Medpace, Pharmalex, Celerion, PRA Health Sciences, Covance (a Labcorp company), and Others |
| Pitfalls & Challenges: | COVID-19 Empact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The rise in chronic diseases and the need for innovative drug therapies fuel the market growth. Additionally, the country’s strong regulatory framework and well-established healthcare infrastructure provide a conducive environment for pharmacokinetics research. Technological advancements, such as high-throughput screening and advanced modeling techniques, enhance the precision and efficiency of studies. Collaboration between pharmaceutical companies, CROs, and research institutions also strengthens the market, fostering innovation and accelerating drug development.
Restraining Factors
Regulatory complexities and lengthy approval processes may slow down the development timeline. Additionally, the shortage of skilled professionals in pharmacokinetics can hinder research productivity.
Market Segmentation
The France Pharmacokinetics Services Market share is classified into drug type and application.
- The small molecules segment held a significant share in 2024 and is expected to grow at a remarkable CAGR during the forecast period.
The France pharmacokinetics services market is segmented by drug type into small molecules and large molecules. Among these, the small molecules segment held a significant share in 2024 and is expected to grow at a remarkable CAGR during the forecast period. The segment is driven due to their widespread use in drug development. Small molecules, which include most traditional drugs, are easier to manufacture, cost-effective, and have a well-established regulatory approval process.
- The SMEs segment held a major market share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The France pharmacokinetics services market is segmented by application into SMEs and large enterprise. Among these, the SMEs segment held a major market share in 2024 and is expected to grow at a significant CAGR during the forecast period. This is attributed to the increasing number of biotech startups and small pharmaceutical companies focusing on innovative drug development. SMEs often collaborate with contract research organizations (CROs) to access specialized pharmacokinetics services, helping them navigate regulatory requirements and optimize drug development processes efficiently.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the France pharmacokinetics services market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Charles River Laboratories
- Laboratory Corporation of America (Labcorp) Drug Development
- Syneos Health
- Quotient Sciences
- Eurofins Scientific
- Medpace
- Pharmalex
- Celerion
- PRA Health Sciences
- Covance (a Labcorp company)
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at France, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the France pharmacokinetics services market based on the below-mentioned segments:
France Pharmacokinetics Services Market, By Drug Type
- Small Molecules
- Large Molecules
France Pharmacokinetics Services Market, By Application
- SMEs
- Large Enterprise
Need help to buy this report?