France Hemophilia Market Size, Share, and COVID-19 Impact Analysis, By Type (Hemophilia A, Hemophilia B, and Others), By Treatment Type (On-demand, Cure, and Prophylaxis), and France Hemophilia Market Insights, Industry Trend, Forecasts to 2035.
Industry: HealthcareFrance Hemophilia Market Insights Forecasts to 2035
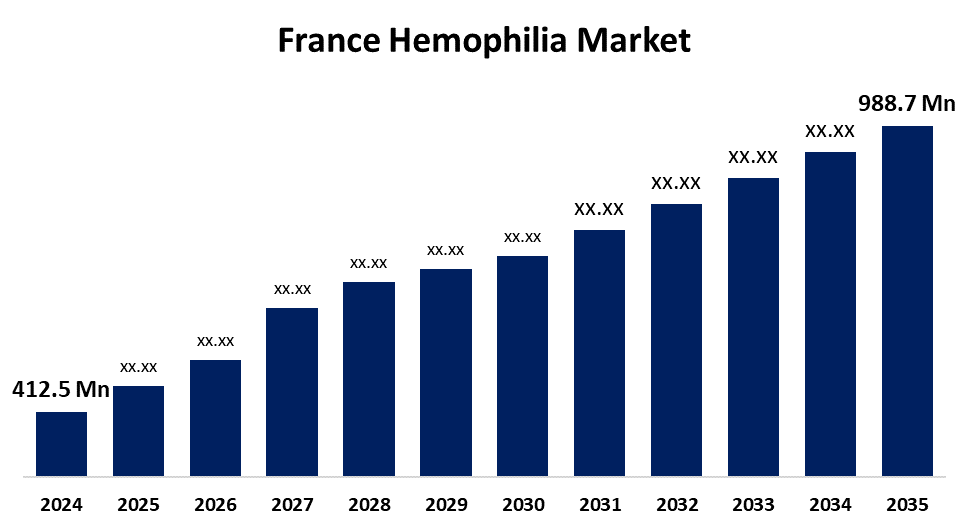
- The France Hemophilia Market Size was estimated at USD 412.5 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 8.27% from 2025 to 2035
- The France Hemophilia Market Size is Expected to Reach USD 988.7 Million by 2035
Get more details on this report -
According to a research report published by Spherical Insights & Consulting, The France Hemophilia Market Size is Anticipated to reach USD 988.7 Million By 2035, Growing at a CAGR of 8.27% from 2025 to 2035. Increasing awareness among people regarding hemophilia disorders and rising government initiatives for early detection in neonates are expected to drive market growth.
Market Overview
The France hemophilia market is the market of products and services for The Treatment and management of hemophilia, a rare genetic bleeding disorder that affects blood clotting. It involves a range of therapies, ranging from replacement therapy to emerging gene therapies, and diagnostic devices and services related to them. There are the following types of hemophilia: A, B, etc. The major market drivers are the approval of gene therapy to treat hemophilia and the presence of several other candidates in various stages of the pipeline. Furthermore, growing awareness about hemophilia disorders and increasing government support programs toward early detection in neonates are anticipated to fuel market growth. The Factors fuelling the haemophilia market growth are the rising incidence of haemophilia, which is boosting demand for treatment options. Severe haemophilia patients need to follow a regular haemophilia treatment regimen to maintain sufficient clotting factors in circulation to prevent bleeding.
Report Coverage
This research report categorizes the market for the France hemophilia market based on various segments and regions and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the France hemophilia market. Recent market developments and competitive strategies such as expansion, product launch, development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the France hemophilia market.
France Hemophilia Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 412.5 Million |
| Forecast Period: | 2025 - 2035 |
| Forecast Period CAGR 2025 - 2035 : | 8.27% |
| 2035 Value Projection: | USD 988.7 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 210 |
| Tables, Charts & Figures: | 100 |
| Segments covered: | By Type, By Treatment Type and COVID-19 Impact Analysis |
| Companies covered:: | Takeda Pharmaceutical Company Limited, Pfizer, Inc., BioMarin, Novo Nordisk A/S., CSL Behring, Sanofi, Spark Therapeutics, Inc., F. Hoffmann La-Roche Ltd., Octapharma AG., Bayer AG, and other key vendors. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The France hemophilia market is driven by the factors driving the haemophilia market growth, is the rising incidence of haemophilia, which is boosting demand for treatment options. Severe haemophilia patients need to follow a regular haemophilia treatment regimen to maintain sufficient clotting factors in circulation to prevent bleeding. The key drivers of the market are the approval of gene therapy for the treatment of hemophilia and the availability of several other candidates at various stages of the pipeline. Additionally, increasing awareness among people regarding hemophilia disorders and rising government initiatives for early detection in neonates are expected to drive market growth.
Restraining Factors
The France hemophilia market is restrained owing to the absence of specific local and regional data, this review does not incorporate region- or country-specific costs. In addition, it does not report elaborate use details of pdFVIII/IX, rFVIII/IX, mimetics, or bypassing agents in the region. This is owing to a lack of adequate, well-documented evidence.
Market Segmentation
The France hemophilia market share is classified into type and treatment type.
- The hemophilia A segment held the highest share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The France hemophilia market is segmented by type into hemophilia A, hemophilia B, and others. Among these, the hemophilia A segment held the highest share in 2024 and is expected to grow at a significant CAGR during the forecast period. It is a genetic condition that results in a deficiency of blood clotting factor VIII. The causes of the dominance are the prevalence of hemophilia A being high in developed markets and favorable government policies for product launches in these markets.
- The prophylaxis segment held the largest share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The France hemophilia market is segmented by treatment type into on-demand, cure, and prophylaxis. Among these, the prophylaxis segment held the largest share in 2024 and is expected to grow at a significant CAGR during the forecast period. Prophylaxis treatment is usually given to severe patients. A new treatment strategy for regular infusion of clotting factor concentrates is contributing to segment growth. For example, expansion in the use of the bi-specific antibody product Helimbra (emicizumab) to treat the lack of clotting factor FVIII is driving the market growth.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the France hemophilia market, along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Takeda Pharmaceutical Company Limited
- Pfizer, Inc.
- BioMarin
- Novo Nordisk A/S.
- CSL Behring
- Sanofi
- Spark Therapeutics, Inc.
- F. Hoffmann La-Roche Ltd.
- Octapharma AG.
- Bayer AG
- Others
Recent Development
- In July 2024, Hemgenix (etranacogene dezaparvovec), the first gene therapy to be approved for hemophilia B, was safely administered to two patients in France, the first time the treatment has been administered in a real-world environment in Europe.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at the global, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the France hemophilia market based on the below-mentioned segments:
France Hemophilia Market, By Type
- Hemophilia A
- Hemophilia B
- Others
France Hemophilia Market, By Treatment Type
- On-demand
- Cure
- Prophylaxis
Need help to buy this report?