France Electroencephalography Devices Market Size, Share, and COVID-19 Impact Analysis, By Product (32-Channel, Multichannel, and Others), By Type (Portable, Standalone, and Wearable), and France Electroencephalography Devices Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareFrance Electroencephalography Devices Market Insights Forecasts to 2035
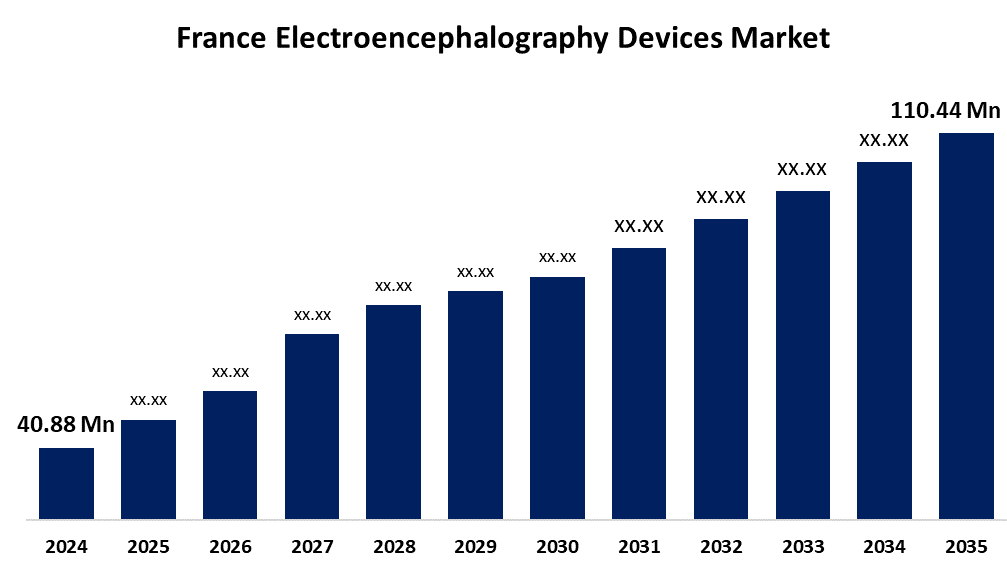
- The France Electroencephalography Devices Market Size was Estimated at USD 40.88 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 9.46% from 2025 to 2035
- The France Electroencephalography Devices Market Size is Expected to Reach USD 110.44 Million by 2035
Get more details on this report -
The France Electroencephalography Devices Market Size is Anticipated to Reach USD 110.44 Million by 2035, Growing at a CAGR of 9.46% from 2025 to 2035. Rising neurological disorders, aging population, and advancements in EEG technology drive France’s EEG devices market growth.
Market Overview
The France electroencephalography (EEG) devices market focuses on the production, distribution, and use of medical equipment designed to record electrical activity in the brain. EEG devices are essential diagnostic tools used in clinical settings to monitor brain function and detect abnormalities such as seizures, brain injuries, and other neurological conditions. These systems work by placing electrodes on the scalp to capture brain wave patterns, which are then analyzed by healthcare professionals. In France, EEG devices are widely used in hospitals, specialized neurology clinics, and research centers. The market includes various types of devices, such as standalone systems, portable units, and advanced digital EEG machines. EEG technology also plays a vital role in sleep studies and cognitive research. The market structure includes both domestic manufacturers and international suppliers, with growing attention on improving accuracy, usability, and patient comfort. The continued development and application of EEG tools make them a critical component of neurological care in France.
Report Coverage
This research report categorizes the market for the France electroencephalography devices market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the France electroencephalography devices market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the France electroencephalography devices market.
France Electroencephalography Devices Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 40.88 Million |
| Forecast Period: | 2024-2035 |
| Forecast Period CAGR 2024-2035 : | 9.46% |
| 2035 Value Projection: | USD 110.44 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 160 |
| Tables, Charts & Figures: | 130 |
| Segments covered: | By Product, By Type, and COVID-19 Impact Analysis. |
| Companies covered:: | Medtronic plc, Natus Medical Incorporated, Bioserenity, GE Healthcare, Philips, Brain Products and Others. |
| Pitfalls & Challenges: | COVID-19 Impact, Challenges, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The increasing incidence of neurological disorders such as epilepsy, Alzheimer’s disease, and Parkinson’s disease has significantly boosted the demand for EEG diagnostics. France’s aging population further contributes to the need for regular brain activity monitoring. Technological advancements, including the development of portable and wireless EEG systems, have improved accessibility and patient comfort. Growing awareness of mental health and neurological conditions, along with expanding healthcare infrastructure and government initiatives, also supports market growth. Additionally, the integration of artificial intelligence in EEG interpretation enhances diagnostic accuracy and clinical efficiency.
Restraining Factors
The complexity of interpreting EEG data requires skilled professionals, and the shortage of trained neurologists and technicians hampers widespread adoption. Regulatory challenges, such as stringent medical device approval processes and compliance with the Medical Device Regulation (MDR), can delay product launches and market access. Furthermore, limited reimbursement coverage for EEG procedures restricts affordability and accessibility for patients.
Market Segmentation
The France electroencephalography devices market share is classified into product and type.
- The 32-channel segment held a leading revenue share in 2024 and is expected to grow at a substantial CAGR during the forecast period.
The France electroencephalography devices market is segmented by product into 32-channel, multichannel, and others. Among these, the 32-channel segment held a leading revenue share in 2024 and is expected to grow at a substantial CAGR during the forecast period. The segment dominance is largely due to their ability to provide detailed brain activity recordings while maintaining ease of use and affordability. These systems strike a balance between basic and highly advanced models, making them ideal for routine clinical use, especially in hospitals and neurology clinics.
- The standalone segment held a major revenue share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The France electroencephalography devices market is segmented by type into portable, standalone, and wearable. Among these, the standalone segment held a major revenue share in 2024 and is expected to grow at a significant CAGR during the forecast period. The segment is driven because standalone EEG devices are widely used in hospitals and specialized diagnostic centers due to their advanced capabilities, high signal quality, and comprehensive monitoring features. These systems are often preferred for detailed neurological assessments and long-term studies.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the France electroencephalography devices market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Medtronic plc
- Natus Medical Incorporated
- Bioserenity
- GE Healthcare
- Philips
- Brain Products
- Others
Recent Developments:
- In November 2022, EMOTIV partnered with X-trodes to develop a solution for simultaneous brain and physiological measurement, expanding EEG applications beyond laboratory settings.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at France, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the France electroencephalography devices market based on the below-mentioned segments:
France Electroencephalography Devices Market, By Product
- 32-Channel
- Multichannel
- Others
France Electroencephalography Devices Market, By Type
- Portable
- Standalone
- Wearable
Need help to buy this report?