France Contract Research Organization Market Size, Share, and COVID-19 Impact Analysis, By Type (Drug Discovery and Clinical Development), By Clinical Trial (Preclinical Trials, Phase I, Phase II, Phase III, Phase IV, and Others), and France Contract Research Organization Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareFrance Contract Research Organization Market Insights Forecasts to 2035
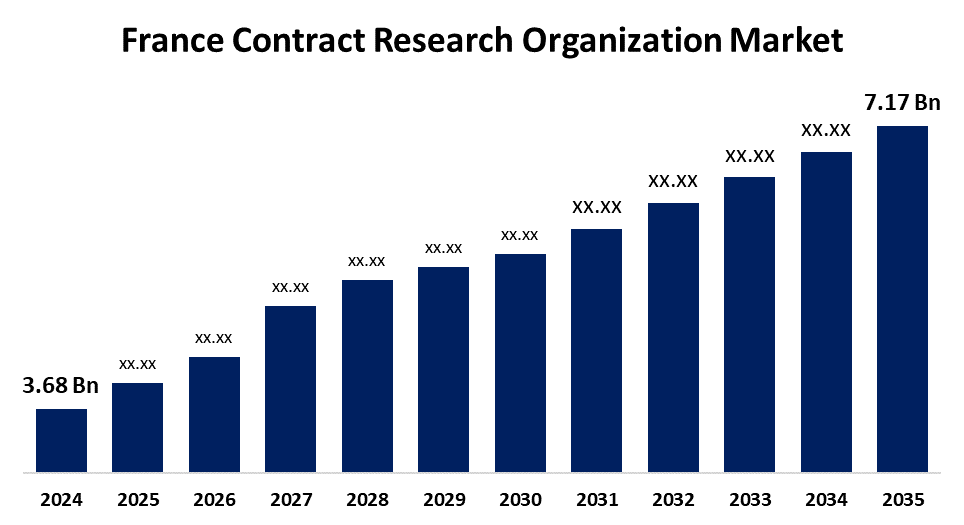
- The France Contract Research Organization Market Size was Estimated at USD 3.68 Billion in 2024
- The Market Size is Expected to Grow at a CAGR of around 6.25% from 2025 to 2035
- The France Contract Research Organization Market Size is Expected to Reach USD 7.17 Billion by 2035
Get more details on this report -
According to a Research Report Published by Spherical Insights & Consulting, The France Contract Research Organization Market Size is anticipated to Reach USD 7.17 Billion by 2035, Growing at a CAGR of 6.25% from 2025 to 2035. Driven by the growing need for clinical research that is outsourced, government programs that encourage innovation, and the growing use of cutting-edge technologies like artificial intelligence and big data analytics.
Market Overview
The market for contract research organizations (CROs) in France is the sector that offers pharmaceutical, biotechnology, and medical device companies outsourced research services. Clinical trial management, regulatory assistance, data analysis, and quality control are some of these services. Additionally, the French government is committed to supporting research and innovation through a variety of programs, creating an environment that is favorable for CROs to thrive. Because of this support, there are now more specialized services that cater to the unique needs of the local market, like regulatory support and clinical trial management. Exploration of this market is possible, especially by utilizing cutting-edge technologies like big data analytics and artificial intelligence. By improving patient recruitment techniques and greatly increasing trial efficiency, these technologies address a significant clinical research challenge. Further, the growing emphasis on personalized medicine in France raises the demand for more specialized research solutions, giving CROs the chance to develop niche products that satisfy consumer needs. Recent patterns show that CROs and academic institutions in France are increasingly working together to foster innovation and knowledge sharing. This cooperative approach accelerates the development of novel treatments and enhances the capabilities of contract research organizations.
Report Coverage
This research report categorizes the market for the France contract research organization market based on various segments and regions and forecasts revenue growth and analyses trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the France contract research organization market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the France contract research organization market.
France Contract Research Organization Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 3.68 Billion |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | CAGR of 6.25% |
| 2035 Value Projection: | USD 7.17 Billion |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 190 |
| Tables, Charts & Figures: | 100 |
| Segments covered: | By Type, By Clinical Trial |
| Companies covered:: | Biotrial, Eurofins Optimed, Clin4all, ExperTrials, ICTA, Oncodesign Services, and Other Key Companies. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
In the French contract research organization market, where customized treatments are becoming more and more important, the drive towards personalized medicine is a significant driver. By 2025, more than 40% of trials in France are expected to be in personalized medicine, according to the French national institute of health and medical research. Major pharmaceutical companies like Ipsen and Servier now rely on CROs that specialize in biomarker identification and diagnostic testing as a result of this growing emphasis. Several CROs in the area provide advanced data analytics and clinical expertise, which are essential for the quickly changing patient-centric model. As a result, they are crucial in supporting the wave of personalized medicine.
Restraining Factors
High operating expenses, intricate regulatory requirements, and restricted access to a variety of patient populations are some of the factors impeding the growth of the contract research organization market in France. Furthermore, streamlined research execution and scalability are hampered by fragmented collaboration between academic and industry stakeholders and competition from global CROs.
Market Segmentation
The France contract research organization market share is classified into type and clinical trial.
- The clinical development segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The France contract research organization market is segmented by type into drug discovery and clinical development. Among these, the clinical development segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period. clinical development is essential since it includes a variety of procedures to evaluate the efficacy and safety of novel medications in people. Thorough regulatory review characterizes this phase, guaranteeing that every product satisfies the requirements to be put on the market. The Clinical Development phase is marked by a notable increase in clinical trials carried out in France, which is ascribed to advantageous laws and a strong healthcare system that facilitates patient enrollment.
- The phase III segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The France contract research organization market is segmented by clinical trial into preclinical trials, phase I, phase II, phase III, phase IV, and others. Among these, the phase III segment held a significant share in 2024 and is expected to grow at a significant CAGR during the forecast period. Phase III trials are important because they include a lot of testing to verify efficacy and track side effects, which frequently leads to market approval.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the France contract research organization market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Biotrial
- Eurofins Optimed
- Clin4all
- ExperTrials
- ICTA
- Oncodesign Services
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at France, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the France Contract Research Organization Market based on the below-mentioned segments:
France Contract Research Organization Market, By Type
- Drug Discovery
- Clinical Development
France Contract Research Organization Market, By Clinical Trial
- Preclinical Trials
- Phase I
- Phase II
- Phase III
- Phase IV
- Others
Need help to buy this report?