Asia Pacific Oncology Molecular Diagnostics Market Size, Share, and COVID-19 Impact Analysis, By Type (Breast Cancer, Lung Cancer, and Other Cancer), By Product (Instruments, Reagents, and Others), By Technology (PCR, Sequencing, and Others), and Asia Pacific Oncology Molecular Diagnostics Market Insights, Industry Trend, Forecasts to 2035
Industry: HealthcareAsia Pacific Oncology Molecular Diagnostics Market Insights Forecasts to 2035
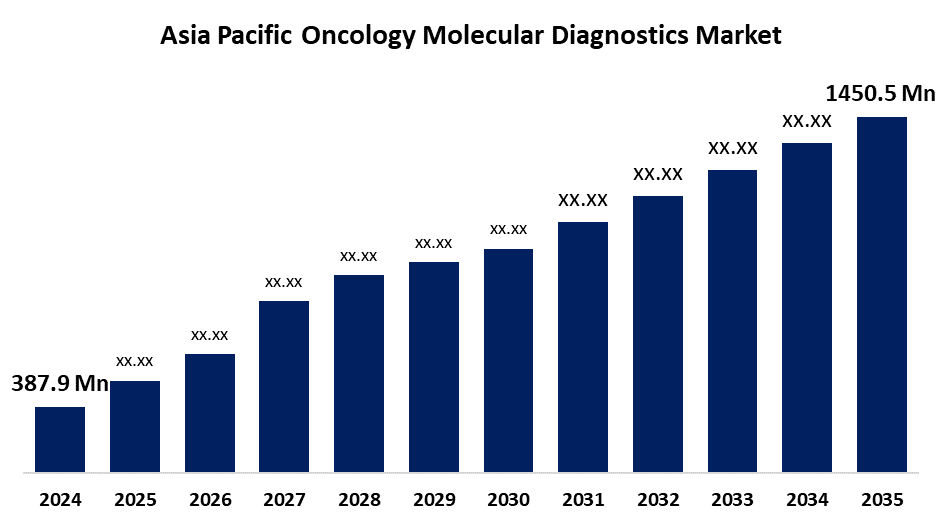
- The Asia Pacific Oncology Molecular Diagnostics Market Size was estimated at USD 387.9 Million in 2024
- The Market Size is Expected to Grow at a CAGR of around 12.74% from 2025 to 2035
- The Asia Pacific Oncology Molecular Diagnostics Market Size is Expected to Reach USD 1450.5 Million by 2035
Get more details on this report -
The Asia Pacific Oncology Molecular Diagnostics Market is anticipated to reach USD 1450.5 million by 2035, growing at a CAGR of 12.74% from 2025 to 2035. The growing cases of cancer, rising healthcare infrastructure, and awareness about personalized medicine are driving the oncology molecular diagnostics market in the Asia Pacific region.
Market Overview
The Asia Pacific oncology molecular diagnostics market is an industry emphasizing the use of molecular techniques for detecting and analyzing cancer-related biomarkers. Cancer molecular diagnostics include tests that are designed to improve the diagnosis and treatment of different forms of cancer (leukemia, lymphoma, and solid tumours). The presence of a well-established regulatory framework, along with significant investments in precision medicine in the region, results in driving the molecular diagnostics demand. There are ongoing research and development activities for discovering new biomarkers and enhancing diagnostic technologies. Further, collaborations between research institutes, pharmaceutical companies, and diagnostic technology are driving the continuous innovation in the oncology molecular diagnostics market. The emergence of precision medicine treatment, which is based on the individual’s genetic characteristics, is driving the need for specialized diagnostic tools, which are offering market growth opportunities.
Report Coverage
This research report categorizes the market for the Asia Pacific oncology molecular diagnostics market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Asia Pacific oncology molecular diagnostics market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Asia Pacific oncology molecular diagnostics market.
Asia Pacific Oncology Molecular Diagnostics Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2024 |
| Market Size in 2024: | USD 387.9 Million |
| Forecast Period: | 2025-2035 |
| Forecast Period CAGR 2025-2035 : | 12.74% |
| 2035 Value Projection: | USD 1450.5 Million |
| Historical Data for: | 2020-2023 |
| No. of Pages: | 210 |
| Tables, Charts & Figures: | 124 |
| Segments covered: | By Type, By Product, By Technology and COVID-19 Impact Analysis |
| Companies covered:: | Abbott Laboratories, F. Hoffmann La Roche Ltd, Siemens Healthineers AG, Danaher Corporation, Thermo Fisher Scientific Inc., Agilent Technologies Inc., Qiagen N.V, Illumina, Inc, Sysmex, Guardant Health, Lucence, LifeStrands, ACT Genomics, Others. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
The increasing prevalence of cancer is responsible for driving the market demand for oncology molecular diagnostics. Cancer incidence in Asia was found to be 169.1 per 100000, which is 49.3% of the global cancer incidence. Further, the rising healthcare infrastructure, with an increasing people's healthcare needs, especially in countries like China, is contributing to propelling the regional market growth. There is an upsurge in the implementation of personalized, targeted therapies in medical oncology. The advancement in molecular diagnostics in cancer pathophysiology is contributing to propelling the market growth.
Restraining Factors
The complex regulatory compliance procedures for product approval and commercialization, as well as the increased cost of molecular diagnostic testing, are limiting the market growth. Further, the sophisticated facility required in the oncology molecular diagnostic testing is challenging the market.
Market Segmentation
The Asia Pacific oncology molecular diagnostics market share is classified into type, product, and technology.
- The breast cancer segment held the largest market revenue share in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Asia Pacific oncology molecular diagnostics market is segmented by type into breast cancer, lung cancer, and other cancer. Among these, the breast cancer segment held the largest market revenue share in 2024 and is expected to grow at a significant CAGR during the forecast period. Molecular diagnostics, including tumor genomic profiling, are increasingly used for breast cancer management. The large test volumes and an extensive portfolio of commercialized products are contributing to driving the oncology molecular diagnostics market growth.
- The reagents segment held the largest share of the oncology molecular diagnostics market in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Asia Pacific oncology molecular diagnostics market is segmented by product into instruments, reagents, and others. Among these, the reagents segment held the largest share of the oncology molecular diagnostics market in 2024 and is expected to grow at a significant CAGR during the forecast period. An increasing utilization of reagents for conducting tests and diagnostics in cancer research, along with expansion in molecular biology technology, is contributing to driving the oncology molecular diagnostics market.
- The PCR segment held the largest revenue share of the oncology molecular diagnostics market in 2024 and is expected to grow at a significant CAGR during the forecast period.
The Asia Pacific oncology molecular diagnostics market is segmented by technology into PCR, sequencing, and others. Among these, the PCR segment held the largest revenue share of the oncology molecular diagnostics market in 2024 and is expected to grow at a significant CAGR during the forecast period. RT-PCR has emerged as a powerful molecular tool for revolutionizing the RT-PCR-based oncology detection by offering precise detection and quantification of cancer-specific biomarkers at the RNA level.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Asia Pacific oncology molecular diagnostics market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Abbott Laboratories
- F. Hoffmann-La Roche Ltd
- Siemens Healthineers AG
- Danaher Corporation
- Thermo Fisher Scientific Inc.
- Agilent Technologies Inc.
- Qiagen N.V
- Illumina, Inc
- Sysmex
- Guardant Health
- Lucence
- LifeStrands
- ACT Genomics
- Others
Recent Developments:
- In September 2023, Guardant Health, Inc., a leading precision oncology company, announced the Japanese Ministry of Health, Labour and Welfare (MHLW) has approved the Guardant360 CDs liquid biopsy test.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at Asia Pacific, regional, and country levels from 2020 to 2035. Spherical Insights has segmented the Asia Pacific Oncology Molecular Diagnostics Market based on the below-mentioned segments:
Asia Pacific Oncology Molecular Diagnostics Market, By Type
- Breast Cancer
- Lung Cancer
- Other Cancer
Asia Pacific Oncology Molecular Diagnostics Market, By Product
- Instruments
- Reagents
- Others
Asia Pacific Oncology Molecular Diagnostics Market, By Technology
- PCR
- Sequencing
- Others
Need help to buy this report?