Asia Pacific In Vitro Diagnostics (IVD) Market Size, Share, and COVID-19 Impact Analysis, By Product Type (Instruments and Reagents & Consumables), By Technique (Immunodiagnostics, Clinical Chemistry, Molecular Diagnostics, Hematology, and Others), By Application (Infectious Diseases, Cardiology, Oncology, Gastroenterology, and Others), By End-user (Clinical Laboratories, Hospitals, Physicians Offices, and Others), and Asia Pacific In Vitro Diagnostics (IVD) Market Insights, Industry Trend, Forecasts to 2032
Industry: HealthcareAsia Pacific In Vitro Diagnostics (IVD) Market Insights Forecasts to 2032.
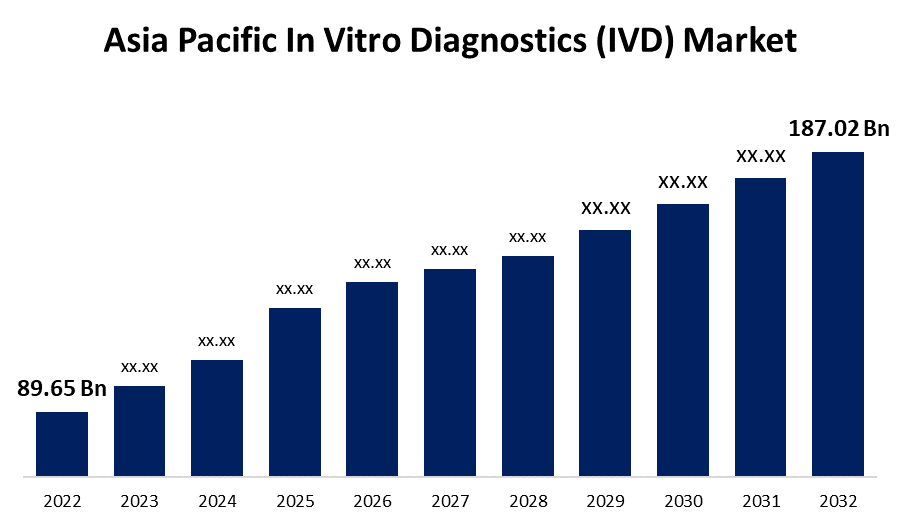
- The Asia Pacific In Vitro Diagnostics (IVD) Market Size was valued at USD 89.65 Billion in 2022.
- The Market is Growing at a CAGR of 7.6% from 2022 to 2032.
- The Asia Pacific In Vitro Diagnostics (IVD) Market is expected to reach USD 187.02 Billion by 2032.
Get more details on this report -
The Asia Pacific In Vitro Diagnostics (IVD) Market Size was valued at USD 89.65 Billion in 2022. The Asia Pacific In Vitro Diagnostics (IVD) Market Size is projected to exceed USD 187.02 Billion by 2032, Growing at a CAGR of 7.6% from 2022 to 2032.
Market Overview
The Asia Pacific In Vitro Diagnostics (IVD) market is a fast-growing and rapidly expanding sector of the healthcare industry, encompassing a wide range of medical tests and diagnostic procedures performed outside of the human body. IVD is a broad category of techniques and products used to analyze biological specimens such as blood, urine, tissue, and other bodily fluids in order to detect diseases, monitor health conditions, and guide medical treatment decisions. This burgeoning Asia Pacific market is distinguished by its vast geographic diversity, varying healthcare infrastructure, and a growing middle-class population with increasing healthcare awareness. Clinical chemistry, molecular diagnostics, immunoassays, point-of-care testing, microbiology, and hematology are some of the IVD tests and instruments available. These diagnostics play an important role in disease prevention, early detection, and personalized medicine, resulting in increased demand from healthcare providers, laboratories, and patients.
Report Coverage
This research report categorizes the market for Asia Pacific In Vitro Diagnostics (IVD) market based on various segments and regions and forecasts revenue growth and analyzes trends in each submarket. The report analyses the key growth drivers, opportunities, and challenges influencing the Asia Pacific In Vitro Diagnostics (IVD) market. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger, and acquisition have been included to draw the competitive landscape in the market. The report strategically identifies and profiles the key market players and analyses their core competencies in each sub-segment of the Asia Pacific In Vitro Diagnostics (IVD) market.
Asia Pacific In Vitro Diagnostics (IVD) Market Report Coverage
| Report Coverage | Details |
|---|---|
| Base Year: | 2022 |
| Market Size in 2022: | USD 89.65 Billion |
| Forecast Period: | 2022-2032 |
| Forecast Period CAGR 2022-2032 : | 7.6% |
| 2032 Value Projection: | USD 187.02 Billion |
| Historical Data for: | 2018-2021 |
| No. of Pages: | 200 |
| Tables, Charts & Figures: | 120 |
| Segments covered: | By Product Type, By Technique, By Application, By End-user and COVID-19 Impact Analysis. |
| Companies covered:: | Shimadzu Corporation, NIHON KOHDEN CORPORATION, Nipro, Sysmex Corporation, DiaSorin S.p.A., Danaher Corporation, bioMérieux SA and other ket vendors. |
| Pitfalls & Challenges: | COVID-19 Empact, Challenge, Future, Growth, & Analysis |
Get more details on this report -
Driving Factors
Chronic diseases such as diabetes, cardiovascular disease, cancer, and infectious diseases are becoming more common in the Asia Pacific region. As the prevalence of these diseases rises, so does the demand for IVD tests to enable early detection, disease monitoring, and effective treatment planning. The demographic profile of the region is shifting toward an aging population. Because the elderly are more vulnerable to various health conditions, there is a greater demand for diagnostic tests for age-related diseases. This demographic shift is expected to significantly fuel IVD market growth. Individuals can now access more comprehensive healthcare services due to rising disposable incomes and healthcare spending. This trend leads to increased demand for diagnostic tests, resulting in a favorable market environment for IVD manufacturers and providers.
Restraining Factors
Variability in regulatory frameworks across Asia Pacific countries can stymie market growth. For IVD manufacturers, navigating complex and evolving regulations for product approvals and quality standards can be time-consuming and costly. In some countries, inconsistent or inadequate reimbursement policies for IVD tests can limit patients' access to advanced diagnostics. IVD companies may face difficulties in competitively pricing their products and ensuring widespread adoption.
Market Segment
The Asia Pacific In Vitro Diagnostics (IVD) Market share is segmented into product type and technique.
- The Instruments segment is expected to hold significant share of the Asia Pacific In Vitro Diagnostics (IVD) market during the forecast period.
The Asia Pacific In Vitro Diagnostics (IVD) market is divided by product type into Instruments and Reagents & Consumables. Among these, the Instruments segment is expected to hold significant share of the Asia Pacific In Vitro Diagnostics (IVD) market during the forecast period. The machinery, equipment, and automated systems used to perform diagnostic tests on biological specimens are referred to as instruments in the IVD market. Analyzers, readers, and testing platforms that automate and streamline the diagnostic process are examples of these.
- The Immunodiagnostics segment accounted for significant share of the Asia Pacific In Vitro Diagnostics (IVD) market in 2022.
Based on the technique, the Asia Pacific In Vitro Diagnostics (IVD) market is classified into Immunodiagnostics, Clinical Chemistry, Molecular Diagnostics, Hematology, and Others. Among these, the Immunodiagnostics segment accounted for significant share of the Asia Pacific In Vitro Diagnostics (IVD) market in 2022. Immunodiagnostics is frequently one of the region's largest segments, owing to its wide range of applications. Clinical Chemistry is also important because it is commonly used in healthcare settings. Molecular diagnostics is rapidly growing in popularity, particularly in response to emerging infectious diseases and personalized medicine trends.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the Asia Pacific In Vitro Diagnostics (IVD) market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.
List of Key Companies
- Shimadzu Corporation
- NIHON KOHDEN CORPORATION
- Nipro
- Sysmex Corporation
- DiaSorin S.p.A.
- Danaher Corporation
- bioMérieux SA
- Others
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting and Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at, regional, and country levels from 2019 to 2032. Spherical Insights has segmented the Asia Pacific In Vitro Diagnostics (IVD) Market based on the below-mentioned segments:
Asia Pacific In Vitro Diagnostics (IVD) Market, By Product Type
- Instruments
- Reagents & Consumables
Asia Pacific In Vitro Diagnostics (IVD) Market, By Technique
- Immunodiagnostics
- Clinical Chemistry
- Molecular Diagnostics
- Hematology
- Others
Asia Pacific In Vitro Diagnostics (IVD) Market, By Application
- Infectious Diseases
- Cardiology
- Oncology
- Gastroenterology
- Others
Asia Pacific In Vitro Diagnostics (IVD) Market, By End-user
- Clinical Laboratories
- Hospitals
- Physicians Offices
- Others
Need help to buy this report?