Global Medical Device Regulatory Affairs Market Size To Worth USD 10.48 Billion by 2033 | CAGR of 8.70%
Category: HealthcareGlobal Medical Device Regulatory Affairs Market Size To Worth USD 10.48 Billion by 2033
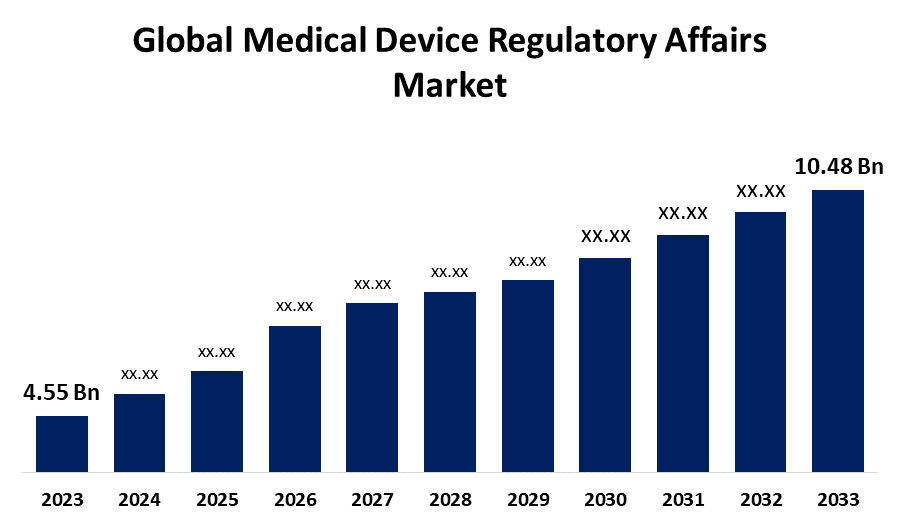
According to a research report published by Spherical Insights & Consulting, the Global Medical Device Regulatory Affairs Market Size is to Grow from USD 4.55 Billion in 2023 to USD 10.48 Billion by 2033, at a Compound Annual Growth Rate (CAGR) of 8.70% during the projected period.
Get more details on this report -
Browse key industry insights spread across 261 pages with 120 Market data tables and figures & charts from the report on the "Global Medical Device Regulatory Affairs Market Size, Share, and COVID-19 Impact Analysis, By Regulatory Phase (Pre-Market, Post-Market), By Service (Product Registration & Clinical Trial Applications, Regulatory Consulting, Legal Representation, Regulatory Writing & Publishing) By Type (Therapeutics and Diagnostic), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 – 2033." Get Detailed Report Description Here: https://www.sphericalinsights.com/reports/medical-device-regulatory-affairs-market
Medical device regulatory affairs are important because it ensures that medical devices are safe and effective before they are sold. Getting approval for medical devices in this case is as strictly regulated as the Food & Drug Administration (FDA) in the U.S. or the Medical Device Regulation (MDR) in Europe. One of the biggest advantages of these regulatory bodies is that it helps ensure patient safety by ensuring that medical devices are properly tested and regulatory compliant. This also gives companies a clear path to start selling their products, which can lead to greater customer trust. However, complying with these regulations can be time-consuming and expensive, which can be very problematic for businesses, especially small businesses, as they have to spend a lot of resources to meet these requirements, and adhering to different regulatory guidelines around the world can be complicated.
The post-market segment is anticipated to hold the greatest share of the global medical device regulatory affairs market during the projected timeframe.
Based on the regulatory phase, the global medical device regulatory affairs market is divided into pre-market and post-market. Among these, the post-market segment is anticipated to hold the greatest share of the global medical device regulatory affairs market during the projected timeframe. Regulatory agencies are prioritizing post-market surveillance for safe and effective medical devices, requiring stricter reporting standards. This demand for specialized post-market services is driving growth in the post-market segment.
The regulatory consulting segment is anticipated to grow at the fastest pace in the global medical device regulatory affairs market during the projected timeframe.
Based on the service, the global medical device regulatory affairs market is divided into product registration & clinical trial applications, regulatory consulting, legal representation, and regulatory writing & publishing. Among these, the regulatory consulting segment is anticipated to grow at the fastest pace in the global medical device regulatory affairs market during the projected timeframe. Regulatory agencies are prioritizing post-market surveillance for safe and effective medical devices, resulting in greater reporting requirements. The need for specialist post-market services is propelling expansion in the post-market industry.
The therapeutic segment is anticipated to grow at the fastest pace in the global medical device regulatory affairs market during the projected timeframe.
Based on the type, the global medical device regulatory affairs market is divided into therapeutics and diagnostic. Among these, the therapeutic segment is anticipated to grow at the fastest pace in the global medical device regulatory affairs market during the projected timeframe. Chronic illnesses, aging populations, and medical advancements increase demand for novel therapeutic devices, necessitating regulatory assistance and significant investment in research and development, particularly in oncology, cardiology, and neurology.
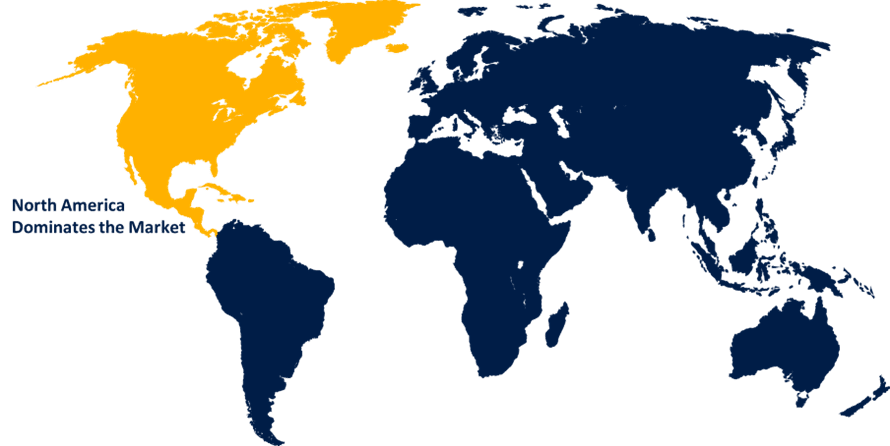
North America is expected to hold the largest share of the global medical device regulatory affairs market over the forecast period.
Get more details on this report -
North America is expected to hold the largest share of the global medical device regulatory affairs market over the forecast period. The US, a major market for medical devices, has a robust regulatory framework, that ensures safety and effectiveness. This environment, led by the FDA, drives innovation and requires expert regulatory affairs services.
Asia Pacific is predicted to grow at the fastest pace in the global medical device regulatory affairs market during the projected timeframe. The region's healthcare and medical device businesses are quickly developing as healthcare costs increase, populations grow, and the general public becomes more aware of new medical technologies. China and India are making significant investments in healthcare infrastructure, raising the need for medical equipment and regulatory support services.
Major vendors in the Global Medical Device Regulatory Affairs Market include TUV SUD, SGS, UL (Underwriters Laboratories), Bureau Veritas, Medpace, PAREXEL, NAMSA, Charles River, Regulatory Compliance Associates, RAPS (Regulatory Affairs Professionals Society), CRB, HCL Technologies, Eurofins Scientific, Catalent, Celerion, and Others.
Recent Developments
- In June 2024, IMed Consultancy published a new white paper examining the regulatory landscape for AI and Machine Learning (ML)--powered medical devices in the United States, United Kingdom, and the European Union.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the Global Medical Device Regulatory Affairs Market based on the below-mentioned segments:
Global Medical Device Regulatory Affairs Market, By Regulatory Phase
- Pre-Market
- Post-Market
Global Medical Device Regulatory Affairs Market, By Service
- Product Registration & Clinical Trial Applications
- Regulatory Consulting
- Legal Representation
- Regulatory Writing & Publishing
Global Medical Device Regulatory Affairs Market, By Type
- Therapeutics
- Diagnostic
Global Medical Device Regulatory Affairs Market, Regional
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
About the Spherical Insights & Consulting
Spherical Insights & Consulting is a market research and consulting firm which provides actionable market research study, quantitative forecasting and trends analysis provides forward-looking insight especially designed for decision makers and aids ROI.
Which is catering to different industry such as financial sectors, industrial sectors, government organizations, universities, non-profits and corporations. The company's mission is to work with businesses to achieve business objectives and maintain strategic improvements.
CONTACT US:
For More Information on Your Target Market, Please Contact Us Below:
Phone: +1 303 800 4326 (the U.S.)
Phone: +91 90289 24100 (APAC)
Email: inquiry@sphericalinsights.com, sales@sphericalinsights.com
Contact Us: https://www.sphericalinsights.com/contact-us
Need help to buy this report?