
World’s Top 50 Real-World Evidence Solutions Market Companies 2025: Industry Intelligence Report by Spherical Insights (2024–2035)
RELEASE DATE: Aug 2025 Author: Spherical InsightsRequest Free Sample Speak to Analyst
Description
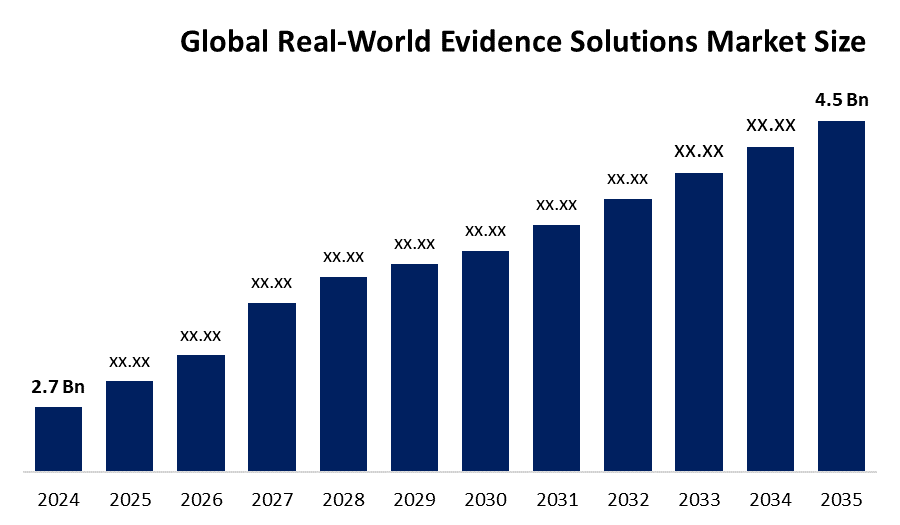
According to a research report published by Spherical Insights & Consulting, The Global Real-World Evidence Solutions Market Size is projected to Grow from USD 2.7 Billion in 2024 to USD 4.5 Billion by 2035, at a CAGR of 4.75% during the forecast period 2025-2035. The market for real-world evidence solutions is in greater demand in Gapan due to its robust real-world data generation is made possible by its vast healthcare data infrastructure, which includes extensive electronic health records, registries, and claims databases.
Introduction
Real-world evidence solutions are sophisticated analytical platforms, tools, and methodologies created to use real-world data (RWD) to obtain clinical insights about the use, efficacy, safety, and value of medical products. Examples of RWD include electronic health records, claims databases, registries, patient-generated data, and wearable device outputs. The use of real-world evidence systems, which have historically been essential for post-approval safety surveillance, is becoming more widespread throughout the whole product lifecycle, including earlier phases of medication development. To expedite the use of RWE in regulatory decision-making, the European Medicines Agency (EMA) has established DARWIN EU, a coordinated network, and released guidelines mandating risk-benefit data in addition to post-authorization safety investigations. Aetion, IQVIA, Flatiron Health, Tempus, and Syapse have united as industry stakeholders to expand the use of RWE among pharmaceutical, device, and regulatory parties. RWE is further improved by technological advancements like AI and IoT, which make it possible to analyse large RWD automatically and in real time. This allows for speedier, data-driven medication discovery and better clinical judgments.
Navigate Future Markets with Confidence: Insights from Spherical Insights LLP
The insights presented in this blog are derived from comprehensive market research conducted by Spherical Insights LLP, a trusted advisory partner to leading global enterprises. Backed by in-depth data analysis, expert forecasting, and industry-specific intelligence, our reports empower decision-makers to identify strategic growth opportunities in fast-evolving sectors. Clients seeking detailed market segmentation, competitive landscapes, regional outlooks, and future investment trends will find immense value in the full report. By leveraging our research, businesses can make ConcertAImed decisions, gain a competitive edge, and stay ahead in the transition toward sustainable and profitable solutions.
Unlock exclusive market insights-Download the Brochure now and dive deeper into the future of the Real-World Evidence Solutions Market.
Real-World Evidence Solutions Market Size & Statistics
- The Market Size for Real-World Evidence Solutions Was Estimated to be worth USD 2.7 Billion in 2024.
- The Market Size is Going to Expand at a CAGR of 4.75% between 2025 and 2035.
- The Global Real-World Evidence Solutions Market Size is anticipated to reach USD 4.5 Billion by 2035.
- The Japan Real-World Evidence Solutions Market Size was valued at USD 89.4 Million in 2023
- The Market is Growing at a CAGR of 9.80% from 2023 to 2033
- The Japan Real-World Evidence Solutions Market Size is Expected to Reach USD 227.6 Million by 2033
- Europe is expected to generate the significant demand during the forecast period in the Real-World Evidence Solutions Market
- Asia Pacific is expected to grow the fastest during the forecast period in the Real-World Evidence Solutions Market.
Regional growth and demand
Asia Pacific is expected to grow the fastest during the forecast period in the Real-World Evidence Solutions market. The market for real-world evidence solutions is now growing in the Asia-Pacific region due to The Asia-Pacific area is emerging as the RWE market with the quickest rate of growth, driven by substantial government support for RWE studies and a significant number of contract research and manufacturing firms in China and India. The increasing need for improved healthcare services is also propelling expansion, even as major market players, including Medpace, Cegedim Health Data, Parexel, IQVIA, and CLINERION LTD., accelerate growth through strategic alliances and product portfolio expansions.
Europe is expected to generate significant demand during the forecast period in the Real-World Evidence Solutions market. The real-world evidence solutions market with the quickest rate of growth throughout the study period is anticipated to be in Europe. The European market for RWE solutions is expanding due to the country's rapidly ageing population and rising rates of hospitalisation for chronic and infectious diseases. Major companies are using strategic initiatives like mergers and product launches to boost growth. Steady market momentum is simultaneously supported by rising R&D investments and growing demand for RWE and RWD.
Top 10 trends in the Real-World Evidence Solutions Market
- Accelerated Regulatory Acceptance
- AI & ML-Powered Analytics
- Diversified & Integrated Data Sources
- Patient-Centric Approaches
- Comparative Effectiveness Research
- Shift Toward Value-and Outcome-Based Care
- Emerging Market Expansion
- Market Consolidation & Collaborations
- Interoperability & Standardization
- Expanded Application Across the Medical Lifecycle
1. Accelerated Regulatory Acceptance
RWE is becoming more and more accepted by regulatory bodies such as the FDA and EMA, which include it in their regulatory submissions and decision-making processes throughout the medication lifecycle. As a result of the 21st Century Cures Act and a 2018 framework, the FDA's RWE program in the United States now permits sponsors to interact with regulators early on regarding RWE protocols and encourages labeling that is grounded in empirical data. The proportion of FDA approvals containing RWE increased significantly from roughly 5-10% to nearly 50% between 2020 and 2024.
2. AI & ML-Powered Analytics
The RWE analytics is undergoing a revolution thanks to artificial intelligence and machine learning, which make it possible to extract more profound and useful insights from large, complicated real-world datasets. Pharmaceutical businesses may anticipate outcomes like disease progression, identify patient subgroups that respond to medicines, and simulate treatment scenarios for better results and comparative efficacy with the use of advanced models, including predictive, probabilistic causal, unsupervised, and deep learning. Clinical applications use AI to improve care protocols based on trends in electronic health data and registries, detect diseases early, and plan individualised treatments. By automating intricate analysis and increasing the accuracy of healthcare data, these technologies improve real-time decision-making in medication development, post-market surveillance, and clinical care.
3. Diversified & Integrated Data Sources
Real-World Evidence is being transformed by the integration of many data sources, which offer a thorough understanding of patient health and treatment results. EHRs, patient registries, insurance claims, wearable technology, social media, and genomic data are important sources of concentration. Regulatory decision-making is improved by initiatives such as the European Medicines Agency's DARWIN EU project, which makes anonymised healthcare data more accessible. The Observational Medical Outcomes Partnership common data model is one example of a standardisation initiative that guarantees compatibility across datasets. This integration makes it possible to evaluate patient outcomes, safety, and treatment efficacy in real-world contexts with greater accuracy.
4. Patient-Centric Approaches
In healthcare, patient-centric approaches place a high priority on patients actively participating in their care, guaranteeing that treatment regimens suit each patient's unique needs, preferences, and values. In order to make educated decisions about their treatment options, patients and healthcare professionals work together with this model's emphasis on shared decision-making. Incorporating patient-reported outcomes and utilising digital health tools like wearable technology and smartphone apps allows physicians to track health data in real time, improving individualised treatment. This strategy not only increases patient satisfaction and treatment plan adherence but also promotes a more engaged and involved patient population, which improves health outcomes.
5. Comparative Effectiveness Research
A key method in healthcare is comparative effectiveness research, which methodically weighs the advantages and disadvantages of various interventions, including drugs, medical equipment, surgeries, and behavioural techniques, to ascertain which is most effective for which patients in particular situations. In contrast to conventional efficacy research, CER emphasises practical efficacy, offering data to guide clinical judgements and enhance patient care. It includes a range of research designs, such as observational studies, systematic reviews, and randomised controlled trials. CER seeks to improve healthcare delivery, optimise treatment approaches, and advance personalised medicine by assessing results in a variety of patient populations. This method helps physicians, patients, and legislators make well-conceived decisions that enhance health outcomes.
Empower your strategic planning:
Stay ConcertAimed with the latest industry insights and market trends to identify new opportunities and drive growth in the Real-World Evidence Solutions market. To explore more in-depth trends, insights, and forecasts, please refer to our detailed report.
Top 25 Companies Leading the Real-World Evidence Solutions Market
- Flatiron Health
- SAS Institute Inc.
- AETION INC.
- Komodo Health
- Medpace Holdings Inc.
- ConcertAI
- Tempus Labs
- Clarivate Plc
- CLINERION LTD.
- VEEVA SYSTEMS
- Verto Health
- PerkinElmer Inc.
- Cegedim Health Data
- Clinigen Group plc
- Elevance Health Inc
- Cognizant Technology Solutions Corporation
- UnitedHealth Group
- IQVIA Inc.
- IBM Corporation
- Optum Inc.
- Oracle Health
- ICON plc
- Syneos Health
- TriNetX LLC
- Thermo Fisher Scientific Inc.
- Flatiron Health
Headquarters: NY, USA
Real-world evidence is being used by Flatiron Health, a renowned health technology company, to change oncology care. To improve cancer treatment, inform policy, and support clinical operations and life sciences research, the organisation compiles and evaluates clinical data from electronic health records and billing systems. Globally, Flatiron supports oncology centres, researchers, and regulators as an autonomous affiliate of Roche. It also keeps offices abroad in places like Europe and Asia to help with its worldwide oncology data projects.
- SAS Institute Inc.
Headquarters: Hamburg, Germany
SAS Institute Inc. is a well-known worldwide supplier of data management software and services, artificial intelligence, and advanced analytics. SAS, SAS Viya, and JMP are among its key products that help businesses manage and analyse large datasets to support decision-making in industries like retail, healthcare, public sector, finance, and life sciences.
- AETION INC.
Headquarters: North Carolina, USA
Aetion Inc. is a healthcare technology company that specialises in real-world evidence solutions. It's an evidence platform that converts real-world data, including electronic health records, claims, and registries, into useful information on the efficacy, safety, and value of treatments. Health technology assessment organisations, payers, regulators, and life sciences firms trust its platform to support important healthcare choices. With tools like Discover, Substantiate, and Generate, Aetion makes it possible to generate transparent, scientifically sound evidence.
- Komodo Health
Headquarters: NY, USA
The healthcare technology business Komodo Health is well-known for its healthcare map, a comprehensive, de-identified, longitudinal data platform that charts patient movements throughout the United States. The platform provides detailed, practical insights to help clinical development, medical affairs, and health economics research through the use of cutting-edge AI and analytics. Its user-friendly tools, such as MapLab and MapView, enable stakeholders to effectively visualise and analyse patient paths, disease trends, and treatment patterns.
- Medpace Holdings Inc.
Headquarters: California, USA
A worldwide full-service clinical contract research organisation with a scientific focus, Medpace Holdings, Inc., provides comprehensive clinical development services for Phase I-IV trials. In a variety of therapeutic areas, such as oncology, cardiology, metabolic and endocrine disorders, central nervous system diseases, and infectious diseases, it provides services to clients in the pharmaceutical, biotechnology, and medical device industries. Medpace simplifies trial execution under one roof with integrated services, including bioanalytical and imaging core labs, clinical monitoring, data administration, regulatory assistance, and medical writing.
Are you ready to discover more about the Real-World Evidence Solutions market?
The report provides an in-depth analysis of the leading companies operating in the global Real-World Evidence Solutions market. It includes a comparative assessment based on their product portfolios, business overviews, geographical footprint, strategic initiatives, market segment share, and SWOT analysis. Each company is profiled using a standardized format that includes:
Company Profiles
- Flatiron Health
- Business Overview
- Company Snapshot
- Products Overview
- Company Market Share Analysis
- Company Coverage Portfolio
- Financial Analysis
- Recent Developments
- Merger and Acquisitions
- SWOT Analysis
- SAS Institute Inc.
- AETION INC.
- Komodo Health
- Medpace Holdings Inc.
- ConcertAI
- Tempus Labs
- Clarivate Plc
- CLINERION LTD.
- Others.
Conclusion
The real-world evidence solutions market is accelerating its market in Japan due to the use of a variety of real-world data, including electronic medical records, claims, registries, and patient-generated insights, to produce solid evidence for clinical and regulatory decision-making. RWE utilisation has been made possible by government and regulatory bodies through programs such as MID-NET and the Clinical Innovation Network, which encourage post-marketing surveillance and the use of external controls in trials. These initiatives tackle growing healthcare demands, ranging from maximising care outcomes and regulatory efficiency to managing an ageing population and the burden of chronic diseases. Japan keeps improving ecosystem development, methodological transparency, and data integration to fully realise the potential of RWE in enhancing patient care.
About the Spherical Insights & Consulting
Spherical Insights & Consulting is a market research and consulting firm which provides actionable market research study, quantitative forecasting and trends analysis provides forward-looking insight especially designed for decision makers and aids ROI.
Which is catering to different industry such as financial sectors, industrial sectors, government organizations, universities, non-profits and corporations. The company's mission is to work with businesses to achieve business objectives and maintain strategic improvements.
CONTACT US:
For More Information on Your Target Market, Please Contact Us Below:
Phone: +1 303 800 4326 (the U.S.)
Phone: +91 90289 24100 (APAC)
Email: inquiry@sphericalinsights.com, sales@sphericalinsights.com
Contact Us: https://www.sphericalinsights.com/contact-us
Need help to buy this report?