
World's Top 50 Companies in Pharmacovigilance and Drug Safety Software in 2025 Watch List: Statistics Report (2024–2035)
RELEASE DATE: Jul 2025 Author: Spherical InsightsRequest Free Sample Speak to Analyst
Description
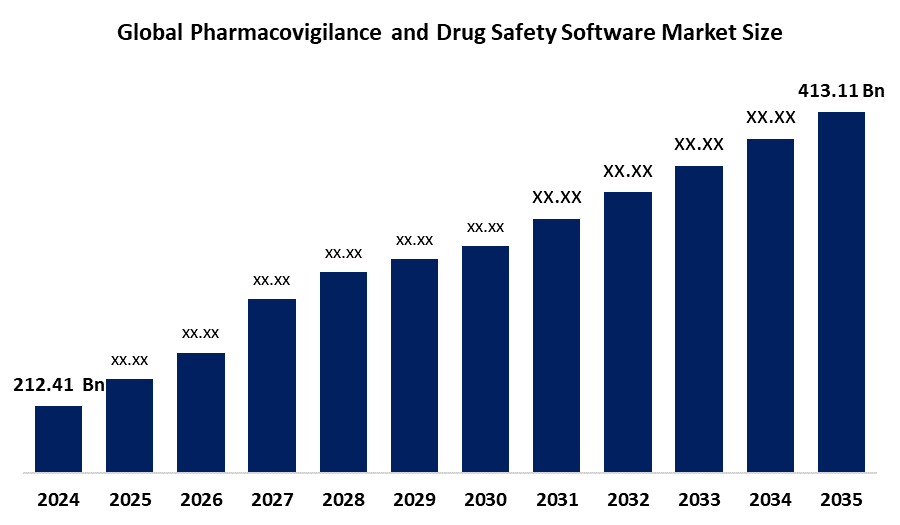
According to a research report published by Spherical Insights & Consulting, The Global Pharmacovigilance and Drug Safety Software Market Size is projected To Grow from USD 212.41 Billion in 2024 to USD 413.11 Billion by 2035, at a CAGR of 6.88 % during the forecast period 2025–2035. The pharmacovigilance and drug safety software market offers future opportunities in AI-driven signal detection, real-time adverse event tracking, regulatory compliance automation, and expanding global demand for efficient drug monitoring systems.
Introduction
The pharmacovigilance and drug safety software market refers to digital solutions used by pharmaceutical companies, contract research organizations, and regulatory bodies to detect, assess, manage, and prevent adverse drug reactions (ADRs). These platforms streamline data collection, automate case processing, and ensure regulatory compliance. Key driving factors include the rising number of adverse drug events, increasing drug consumption, stringent global regulations from agencies like the FDA and EMA, and the growing complexity of clinical trials. Additionally, the demand for real-time safety monitoring, integration with electronic health records (EHR), and AI-powered analytics further propel market growth and adoption worldwide.
Navigate Future Markets with Confidence: Insights from Spherical Insights LLP
The insights presented in this blog are derived from comprehensive market research conducted by Spherical Insights LLP, a trusted advisory partner to leading global enterprises. Backed by in-depth data analysis, expert forecasting, and industry-specific intelligence, our reports empower decision-makers to identify strategic growth opportunities in fast-evolving sectors. Clients seeking detailed market segmentation, competitive landscapes, regional outlooks, and future investment trends will find immense value in the full report. By leveraging our research, businesses can make informed decisions, gain a competitive edge, and stay ahead in the transition toward sustainable and profitable solutions.
Unlock exclusive market insights - Download the Brochure now and dive deeper into the future of the pharmacovigilance and drug safety software market.
Pharmacovigilance and Drug Safety Software Market Size & Statistics
- The Market for Pharmacovigilance and Drug Safety Software was estimated to be worth USD 212.41 Billion in 2024.
- The Market is Going to expand at a CAGR of 6.88 % between 2025 and 2035.
- The Global Pharmacovigilance and Drug Safety Software Market Size is anticipated to reach USD 413.11 Billion by 2035.
- North America is expected to generate the highest demand during the forecast period in the pharmacovigilance and drug safety software Market.
- Asia Pacific is expected to grow the fastest during the forecast period in the pharmacovigilance and drug safety software Market.
Regional growth and demand
Asia Pacific is expected to grow the fastest during the forecast period in the pharmacovigilance and drug safety software market. The Asia Pacific region is expected to have the highest CAGR during the forecast period. Businesses in the Asia Pacific region offer cost-saving advantages, promoting increased clinical trials alongside a focus on pharmacovigilance and drug safety software. Awareness of public safety, stringent government regulations, and a rise in negative drug reactions have led to the widespread recognition of outsourcing centers such as Singapore, South Korea, and Taiwan.
North America is expected to generate the highest demand during the forecast period in the pharmacovigilance and drug safety software market. North America is expected to maintain the largest portion of the worldwide pharmacovigilance and drug safety software market throughout the forecast period due to government-supported initiatives. The open FDA initiative and the Mini-Sentinel project, which offer active surveillance systems, are anticipated to increase usage rates. The rising need for efficient drug safety solutions, stringent regulations, the common occurrence of chronic illnesses, clinical trials, and drug approvals, all supported by numerous CROs offering outsourcing services, are the primary factors behind the region's commercial successes.
Top 5 Emerging Pharmacovigilance Startups Impacting the Industry
- HEPAprint – Adverse Drug Reaction (ADR)
Clinical trials are essential for the process of drug development. Grasping the potential situations regarding drug safety through technological assistance is advantageous for all parties involved. Time plays a crucial role in drug development, as quicker product-to-market timelines enable pharmaceutical companies to enhance profitability. Time dedicated to clinical trials requires investment, and the capacity to anticipate ADRs helps enhance the success rate. UK-based startup HEPAprint creates forecasting software aimed at stopping negative drug responses. Utilizing artificial intelligence (AI) and personal genetic information, the software forecasts the possible impacts of medications on particular genetic markers. Additionally, the software allows doctors to prescribe medications with a greater awareness of potential reactions to enhance overall healthcare services.
- MEDIKURA Digital Health – Post-Market Drug Safety
Even medications available for many years need ongoing surveillance for adverse events and reactions. The progress in digital technologies allows pharma companies to observe specific trends through data collection from end-users. Startups are connecting pharmaceutical companies and patients to address crucial challenges like negative drug effects. The German startup MEDIKURA Digital Health offers digital Software-as-a-Service (SaaS) solutions for pharmacovigilance. The startup facilitates organized data collection from end-users and engages essential participants like pharmacists, doctors, patients, and manufacturers. Consequently, pharmaceutical companies obtain practical insights regarding adverse events or reactions linked to their products in real-time to trigger product recalls or other necessary measures.
- Navro Technology Solutions – AI-Based Pharmacovigilance
The use of artificial intelligence enhances both single case processing and ongoing surveillance of adverse events and drug reactions. Automating repetitive and routine tasks allows for faster reporting and facilitates smooth real-time communication. New companies are creating AI-driven solutions to enhance the productivity of data reporting and the efficiency of the drug development process. Navro Technology Solutions, an Indian startup, offers AI-driven pharmacovigilance solutions. Through deep learning and AI, the startup assists pharmaceutical companies in minimizing the time needed to report adverse drug reactions. NLP and speech & text recognition enhance Navro’s capability to provide accurate reporting of diverse clinical and regulatory details.
- Embleema – Patient Advocacy
Clinical trials for various types of medications frequently require variations in the patient groups. Patient advocacy facilitates the identification of the appropriate patient group and is essential for the success of trials. Technologies like big data and deep learning allow algorithms to comprehend and link genetic markers specific to patients for improved evaluation of potential participants. Analytics solutions that integrate various data parameters and medical logic support patient advocacy to enhance the precision of target identification further. Embleema is a startup located in the US that provides solutions for patient advocacy. Utilizing big data analytics, the startup examines the information gathered from target audiences and provides enhanced insights for the entire medical community. Patient advocacy organizations participate in Embleema Virtual Studies to exchange information regarding diseases and treatment options.
- Cloudbyz – Cloud-Based Pharmacovigilance
With the emergence of digital transformation technologies, collecting, storing, and processing extensive sets of patient-specific data has become straightforward. Consequently, pharmacovigilance gains from the strength of databases for illnesses, medications, and adverse effects and drug responses. Startups and new firms are developing cloud-driven solutions to streamline the analysis of data from various sources. The US company Cloudbyz creates cloud and mobile enterprise applications for pharmacovigilance. The solution combines periodic adverse event reports, patient histories, and business rules including region-specific regulatory standards. Consequently, the solution delivers pharmaceutical companies real-time information to monitor every product in the drug development process.
Empower your strategic planning:
Stay informed with the latest industry insights and market trends to identify new opportunities and drive growth in the pharmacovigilance and drug safety software market. To explore more in-depth trends, insights, and forecasts, please refer to our detailed report.
Top 12 Companies Leading the Pharmacovigilance and Drug Safety Software Market
- IQVIA
- Genpact
- Aris Global
- Accenture
- IBM
- Capgemini
- Paraxel International Corporation
- Cognizant
- United BioSource Corporation
- Ennov Solutions Inc.
- Veeva Systems
- Others
1. IQVIA
Headquarters: Durham, North Carolina, USA
IQVIA is a global leader in health information technology and clinical research. The company provides advanced analytics, technology solutions, and contract research services to the life sciences industry. IQVIA offers robust pharmacovigilance and drug safety platforms that support adverse event reporting, regulatory compliance, and signal detection using AI and real-world data. Its cloud-based technologies and global network of experts enable pharmaceutical companies to manage risks effectively across drug development and post-marketing phases. With deep domain expertise and scalable tech infrastructure, IQVIA plays a crucial role in advancing data-driven drug safety and healthcare innovation.
2. Genpact
Headquarters: New York, New York, USA
Genpact is a global professional services firm focused on delivering digital transformation through data, technology, and domain expertise. In the healthcare and life sciences space, Genpact provides end-to-end pharmacovigilance solutions, including case processing, regulatory reporting, and safety analytics. Leveraging automation, AI, and cloud platforms, Genpact supports clients in maintaining compliance and enhancing drug safety. Their services help pharma companies scale operations efficiently while reducing costs and improving accuracy. With decades of experience and a strong global delivery model, Genpact serves leading pharmaceutical firms and contract research organizations across the pharmacovigilance value chain.
3. ArisGlobal
Headquarters: Waltham, Massachusetts, USA
ArisGlobal is a life sciences software company that develops cloud-based solutions for pharmacovigilance, regulatory affairs, clinical development, and medical affairs. Its flagship platform, LifeSphere®, is widely used for drug safety monitoring, adverse event reporting, signal detection, and compliance. ArisGlobal leverages automation, artificial intelligence, and machine learning to streamline case processing and improve operational efficiency. The company supports pharmaceutical, biotech, and medical device companies in meeting evolving global regulatory requirements. With a customer base spanning over 300 life sciences organizations, ArisGlobal is a key player in digital pharmacovigilance transformation and drug safety innovation.
4. Accenture
Headquarters: Dublin, Ireland
Accenture is a global professional services firm specializing in digital, cloud, and security solutions. It offers comprehensive pharmacovigilance services through its Life Sciences division, including case intake, medical review, signal detection, and regulatory submissions. Accenture integrates AI and automation tools to enhance accuracy and reduce turnaround times in safety operations. The company partners with major pharmaceutical and biotech firms to modernize their drug safety systems and ensure global compliance. Its deep industry knowledge, combined with advanced analytics and scalable delivery capabilities, positions Accenture as a leading partner for end-to-end pharmacovigilance transformation and digital innovation.
5. IBM
Headquarters: Armonk, New York, USA
IBM is a multinational technology and consulting company that provides AI-driven solutions for various industries, including life sciences. In the pharmacovigilance space, IBM Watson Health offers tools for automated case processing, adverse event detection, and signal management. Its analytics and cloud technologies enable pharmaceutical companies to improve decision-making, maintain regulatory compliance, and ensure patient safety. IBM’s systems help streamline pharmacovigilance workflows and integrate safety data from multiple sources. With a legacy of innovation and a strong AI portfolio, IBM plays a vital role in digitalizing drug safety operations for global pharma and healthcare organizations..
Are you ready to discover more about the pharmacovigilance and drug safety software market?
The report provides an in-depth analysis of the leading companies operating in the global pharmacovigilance and drug safety software market. It includes a comparative assessment based on their product portfolios, business overviews, geographical footprint, strategic initiatives, market segment share, and SWOT analysis. Each company is profiled using a standardized format that includes:
Company Profiles
- IQVIA.
- Business Overview
- Company Snapshot
- Products Overview
- Company Market Share Analysis
- Company Coverage Portfolio
- Financial Analysis
- Recent Developments
- Merger and Acquisitions
- SWOT Analysis
- Genpact
- Aris Global
- Accenture
- IBM
- Capgemini
- Paraxel International Corporation
- Cognizant
- United BioSource Corporation
- Ennov Solutions Inc.
- Veeva Systems
- Others
Conclusion
The pharmacovigilance and drug safety software market is evolving rapidly with technological advancements, regulatory pressures, and the increasing complexity of drug development and post-market surveillance. Digital solutions are becoming essential for efficient adverse event tracking, real-time monitoring, and global regulatory compliance. Innovations in artificial intelligence, cloud computing, and big data analytics are enabling smarter, faster, and more reliable safety processes. As pharmaceutical and biotech companies strive for improved patient safety and operational efficiency, investment in advanced drug safety platforms continues to rise. The market holds significant potential, particularly in emerging regions, as stakeholders seek scalable, integrated, and proactive pharmacovigilance solutions.
About the Spherical Insights & Consulting
Spherical Insights & Consulting is a market research and consulting firm which provides actionable market research study, quantitative forecasting and trends analysis provides forward-looking insight especially designed for decision makers and aids ROI.
Which is catering to different industry such as financial sectors, industrial sectors, government organizations, universities, non-profits and corporations. The company's mission is to work with businesses to achieve business objectives and maintain strategic improvements.
CONTACT US:
For More Information on Your Target Market, Please Contact Us Below:
Phone: +1 303 800 4326 (the U.S.)
Phone: +91 90289 24100 (APAC)
Email: inquiry@sphericalinsights.com, sales@sphericalinsights.com
Contact Us: https://www.sphericalinsights.com/contact-us
Need help to buy this report?